On March 28th, the newly upgraded China Innovative Medical Devices and Drugs Exhibition opened grandly at the "Never Ending" International Innovative Medical Devices Exhibition in Boao Lecheng. This is the first external window in China to showcase the effectiveness of China's drug and medical device review and approval system reform, which has a positive promoting effect on encouraging and supporting the research and development of domestically innovative drugs and medical devices.


In August 2015, the State Council issued the "Opinions on Reforming the Review and Approval System for Pharmaceutical and Medical Devices", marking the beginning of the reform of China's pharmaceutical and medical device review and approval system. Especially since its establishment in 2018, under the strong leadership of the Party Central Committee and the State Council, the National Drug Administration has firmly established the people-centered development concept, continuously deepened the reform of the review and approval system, encouraged innovation in drugs and medical devices, improved the quality of drugs and medical devices, increased effective supply, and effectively ensured the physical health and life safety of the people.


The report of the 20th National Congress of the Communist Party of China proposes to accelerate the implementation of the innovation driven development strategy and achieve high-level technological self-reliance and self-improvement. In order to effectively utilize the advantages of the internationalization platform in Lecheng Pilot Zone, with the strong support and careful guidance of the National Drug Administration, the China Innovation Medical Device and Drug Exhibition has settled at the Boao Lecheng "Never Ending" International Innovation Medical Device Exhibition, becoming an important window to showcase the reform of China's drug and medical device review and approval system at home and abroad, as well as the achievements of the National Drug Administration's encouragement of drug and medical device innovation and development.
Taking advantage of the good opportunity of the opening of the Boao Forum for Asia 2023 Annual Conference, the newly upgraded China Innovative Medical Devices and Drugs Exhibition was officially opened to the outside world. It will give full play to the advantage of Lecheng pilot area as an international window for medical opening up, promote China Innovative Medical Devices and Drugs Exhibition to form a new window for attracting global innovative drugs to exchange and display in Hainan, and release a positive signal to encourage domestic innovative drug and medical device research and development, Fully demonstrate the achievements of the reform of the national drug and medical device review and approval system.
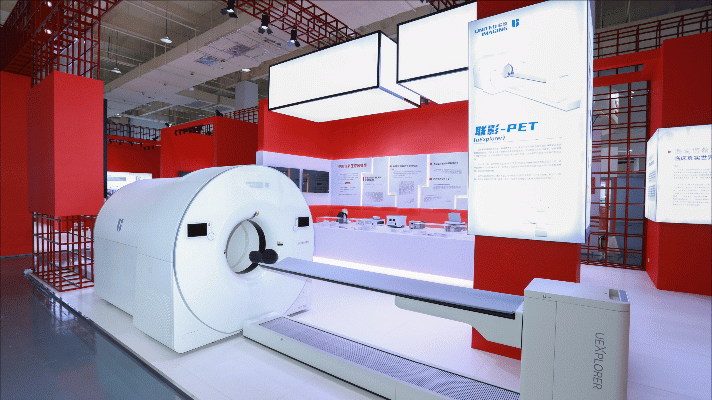
The China Innovative Medical Equipment Exhibition is composed of multiple exhibition areas, including medical imaging, tumor therapy, cardiovascular and cerebrovascular therapy, in vitro diagnosis, orthopedics and dentistry, surgical treatment, household appliances, and the Hainan exhibition area.
The "Carbon Ion Therapy System" developed by Lanzhou Kejin Taiji Company is the first self owned intellectual property carbon ion therapy system approved for listing by the National Drug Administration, breaking the patent barriers of foreign products; Shandong Weigao's Abdominal Endoscopic Surgery System ("Miaoshou" Surgical Robot System) is the first approved endoscopic surgery robot in China, filling the domestic gap; The multi target fecal FIT-DNA detection kit independently developed by Nuohui Health is currently the first and only cancer early screening product approved by the National Drug Administration in China. It has become a recommended technology for all national guidelines for early screening and diagnosis of colorectal cancer; Beitaifu's comprehensive diagnosis and treatment equipment for tinnitus and deafness integrates the detection, diagnosis, and treatment functions of tinnitus and deafness.
China Innovative Medicine Exhibition has set up four exhibition areas, namely, COVID-19 Epidemic Prevention, Chemical Drugs, Biological Agents and Traditional Chinese Medicine Preparations.
Xiannuoxin of Pioneer Pharmaceutical is the first independently developed anti COVID-19 oral drug targeting 3CL protease in China, which is used to treat adult patients with mild to moderate novel coronavirus infection (COVID-19); The total alkaloid tablet of Sangzhi from Beijing Wuhe Boao Pharmaceutical Co., Ltd., used to treat type 2 diabetes, can effectively reduce the glycosylated hemoglobin level of type 2 diabetes subjects; Hualing Pharmaceutical's Dozagliptin tablets are the first domestically produced glucokinase (GK) activator; Rongchang Biological's injectable Taitasipu is a domestically developed dual targeted biological drug targeting BLyS and APRIL, providing a new treatment option for patients with systemic lupus erythematosus.
It is reported that there are a total of 106 innovative medical devices and over 80 innovative drugs exhibited at the China Innovative Medical Device and Drug Exhibition. The participating companies include many well-known domestic brands such as Mindray Medical, Lianying Medical, Lepu Medical, Huada Zhizao, Zaiding Pharmaceutical, Junshi Biological, Zhenzhen Biological, Huabei Pharmaceutical, Anhan Technology, Wobi Medical, Yaoming Junuo, Yiling Pharmaceutical, Haizheng Pharmaceutical, etc.
2023 is the beginning year for the comprehensive implementation of the spirit of the 20th National Congress of the Communist Party of China, and a key year for promoting the closure and operation of Hainan Free Trade Port. With the strong support of the National Food and Drug Administration and the correct leadership of the Hainan Provincial Party Committee and Government, the Hainan Provincial Food and Drug Administration and Lecheng Management Bureau jointly promote the "Never Ending" International Innovative Medicine and Equipment Exhibition to become a gateway for international advanced medicine and equipment to enter China, a window for domestic innovative medicine and medical equipment to be displayed externally, and accelerate the construction of a world-class international medical tourism destination and medical technology innovation platform, Provide more and better guarantees for the health of the people.